Thermodynamics
Basic info (click to expand)
Number of moles
- : molar mass (kg/mol), mass of 1 mole of the substance
- : number of molecules
- mol : Avogadro constant
Pressure
Density
Litter to cubic meter: L = m
Summary
First law of thermodynamics:
| Change in internal energy | Heat | Work done | |
|---|---|---|---|
| Isothermal () | |||
| Isochoric () | |||
| Isobaric () | |||
| Adiabatic () |
Ideal gas law
- J/molK : universal gas constant
- J/K : Boltzmann constant
First law of thermodynamics
- : change in internal energy
- path-independent
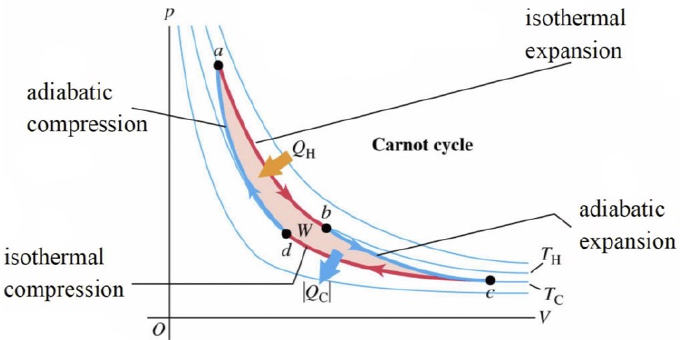
- For a cyclic process,
- : heat enters into system from surroundings
- path-dependent
- : work done by the system
- = Area under p-V curve
- path-dependent
- Expansion:
- Compression:
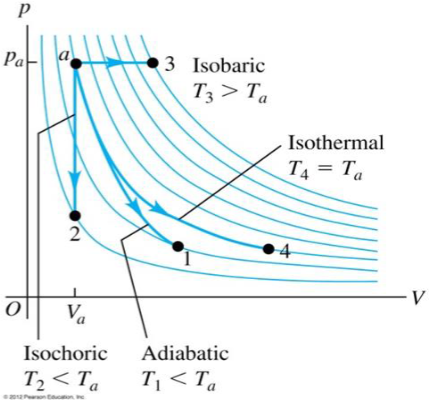
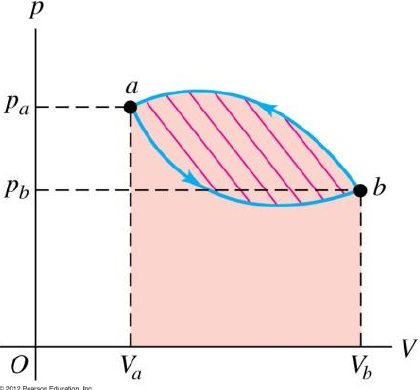
Example cyclic process with
KE of ideal gas
Total translational KE of all molecules:
Root-mean-square speed of molecules:
Most probable speed of molecules:
Average speed of molecules:
Molar heat capacity of ideal gas
At constant volume:
At constant pressure:
Adiabatic index:
Degrees of freedom
Monoatomic gas:
- and
- Translation only
Diatomic gas:
- and
- Translation + rotation
- At very high temperature:
- and
- Translation + rotation + vibration
Adiabatic process
- : no heat enters into or exits from the system
- e.g. free expansion of gas